Photoacid Generator: 6-Butyl-N-hydroxynaphthalimide trifluoromethanesulfonic acid [1610827-31-0]
Time:2023-12-20 Hits:225
6-Butyl-N-hydroxynaphthalimide trifluoromethanesulfonic acid [1610827-31-0] is a cationic photoinitiator and nonionic photoacid generator, among other additives, predominantly utilized for the surface modification of polymer films, and the preparation of positive tone photoresist components, photoresist compositions, and composite materials, including substrates and coatings applied to their surfaces.
Basic Product Information
Product Name: 6-Butyl-N-hydroxynaphthalimide trifluoromethanesulfonic acid, 6-Bu-NIOTf
English Name: 6-Butyl-1,3-dioxo-1H-Benzo[de]Isoquinolin-2(3H)-yl Trifluoromethanesulfonate
CAS No.: 1610827-31-0
Molecular Formula: C17H14F3NO5S
Molecular Weight: 401.36
Specifications: 1G/5G/10G/25G
Application Areas: Electronic chemistry, semiconductor chemistry, photoresist, photogenerator/photoacid/PAG, light curing, UV curing, 3D printing, etc.
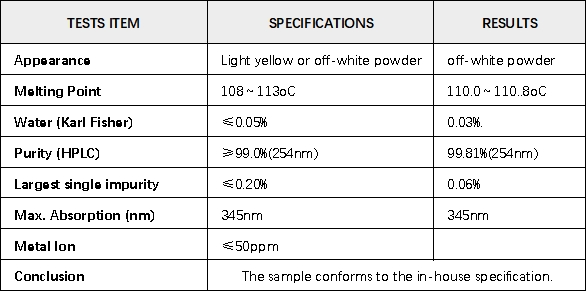
Certificate of Analysis
Product List
|
Item number
|
Product
|
CAS No.
|
|
AAU16172A
|
6-Butyl-N-hydroxynaphthalimide trifluoromethanesulfonic acid,6-Bu-NIOTf
|
1610827-31-0
|
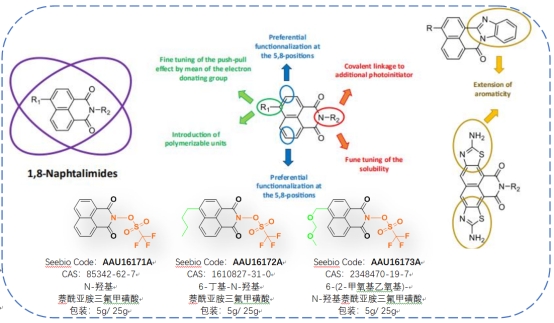
Characteristics of Hydroxynaphthalimide Trifluoromethanesulfonate Photoacid Agent
Acid Production Mechanism: Upon exposure to a suitable light source, the sulfonate ester bond breaks, leading to acid generation. Photoacid generators based on naphthalene imide sulfonate esters exhibit enhanced acid production efficiency, with some naphthalene-structured photoacids being twice as potent as others.
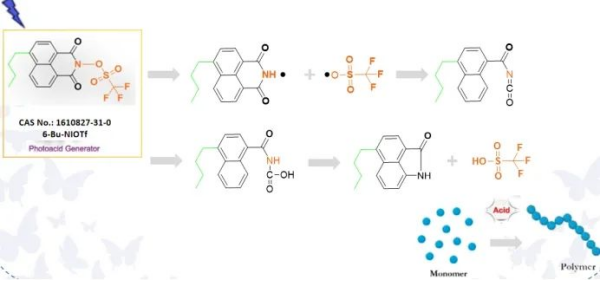
Characteristics of Naphthalimide Acid Generators:
High light absorption energy; excellent solubility in organic solvents; robust thermal stability; rapid acid production rate.
Easy to enhance the properties of the corresponding photoacid by chemically modifying and tailoring the structure. Naphthalene imide naphthalene sulfonate photoacids can be finely tuned through various chemical modifications and tailoring, making them suitable for adjusting solubility, polyaromaticity, or altering the substitution pattern of the naphthalene core. These attributes render them suitable for multifunctional photoinitiation applications.