Cytokines and Their Receptors: Dual Functions in Inflammation and Immune Regulation
Time:2024-11-29 Hits:15
Cytokines constitute a class of low-molecular-weight soluble proteins, typically ranging from 5 to 20 kDa, which are produced by various cells in response to immunogens, mitogens, or other stimulatory signals. Through their interaction with specific receptors on the cell surface, these proteins initiate signal transduction pathways, ultimately inducing alterations in gene transcription within the cell. Cytokines serve diverse roles in regulating innate and adaptive immunity, hematopoiesis, cellular proliferation, the maintenance of multipotent APSC cells, and tissue repair following damage. Based on their primary functions, cytokines can be categorized into several key groups: interleukins (ILs), colony-stimulating factors (CSFs), interferons (IFNs), tumor necrosis factors (TNFs), the transforming growth factor-β (TGF-β) family, growth factors (GFs), and the chemokine family.
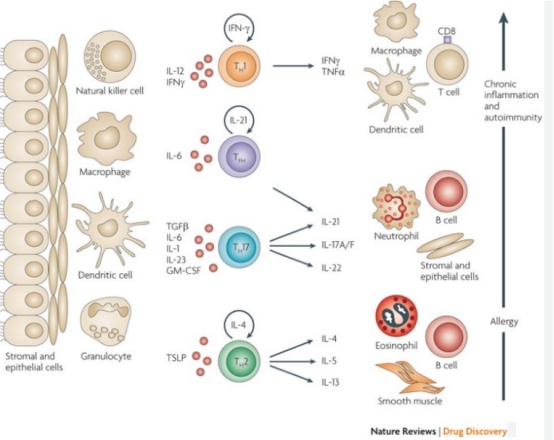
Cytokines are small molecular proteins or polypeptides synthesized and secreted by cells, serving as crucial mediators of intercellular signaling. Their functions primarily encompass immune regulation and effector activities, which include modulating innate and adaptive immune responses, stimulating hematopoiesis, facilitating tissue repair following damage, and regulating cellular growth and differentiation. By finely tuning the intensity and duration of immune responses, cytokines play a pivotal role throughout the immune system.
Within the body, cytokines interact in a highly intricate manner, forming an exceptionally complex immune regulatory network. These specific cytokines exert their biological effects through autocrine, paracrine, or endocrine mechanisms, displaying characteristics such as pleiotropy, functional overlap, antagonism, and synergy. However, under certain circumstances, cytokines can also contribute to the development of various diseases, and in extreme cases, may even trigger cytokine storms and associated syndromes, resulting in multiple organ damage, functional failure, and potentially fatal outcomes.
Most cytokine receptors (CK-R) are heterodimers or multimers consisting of two or more subunits, typically comprising an α chain that specifically binds to the ligand and a β chain involved in signal transduction. Cytokine receptors occur naturally in two forms: membrane-bound cytokine receptors (mCK-R) and soluble cytokine receptors (sCK-R) found in bodily fluids like serum. The diverse biological activities of cytokines are primarily exerted through their binding to mCK-R, while sCK-R holds unique biological significance, with its level fluctuations closely associated with certain diseases.
The intricate and dynamic interplay between cytokines and their receptors modulates chronic inflammation and immunosuppression within the tumor microenvironment (TME), thereby fostering tumor progression and metastasis and undermining treatment efficacy. Following tumors, autoimmune diseases have emerged as another significant area for cytokine-targeted therapy. By inhibiting cytokine function, tissue damage and inflammation in these diseases can be effectively mitigated. With the progression of biopharmaceutical technology, targeted cytokine therapies hold immense potential for treating tumors and autoimmune diseases in the future.
Seebio Products
|
Targeted Molecule
|
Species
|
Host Cells
|
Descriptions
|
|
IL-2
|
Human
|
HEK293
|
Human IL-2 protein, untagged
|
|
Human
|
HEK293
|
Human IL-2 protein, Fc tag
|
|
|
Human
|
HEK293
|
Human IL-2 protein, His tag
|
|
|
Human
|
HEK293
|
Biotinylated human IL-2 protein, His tag
|
|
|
Human
|
HEK293
|
Biotinylated human IL-2 protein, Fc tag
|
|
|
Mouse
|
HEK293
|
Mouse IL-2 protein, His tag
|
|
|
Cynomolgus
|
HEK293
|
Cynomolgus IL-2 protein, His tag
|
|
|
IL-4
|
Human
|
|
Biotinylated human IL-4 R alpha / CD124 protein, His tag
|
|
Human
|
|
Human IL-4 R alpha / CD124 Protein, Fc Tag
|
|
|
Human
|
|
Human IL-4 R alpha / CD124 Protein, His-Tagged
|
|
|
Mouse
|
|
Mouse IL-4 R alpha / CD124 Protein, His-Tagged
|
|
|
Mouse
|
|
Mouse IL-4 R alpha / CD124 Protein, Fc Tag
|
|
|
IL-6
|
Human
|
|
Biotinylated human IL-6 protein, His tag
|
|
Human
|
|
Biotinylated human IL-6 protein, no epitope tag, ultrasensitive, primary amine labeled
|
|
|
Human
|
|
Human IL-6 protein, His tag
|
|
|
Mouse
|
|
Biotinylated mouse IL-6 protein, His tag
|
|
|
Mouse
|
|
Mouse IL-6 protein, His tag
|
|
|
Cynomolgus
|
|
Cynomolgus IL-6 protein, His tag
|
|
|
IL-6R alpha
|
Human
|
|
Biotinylated human IL-6 R alpha / CD126 protein, Fc tag
|
|
Human
|
|
Human IL-6 R alpha / CD126 Protein, Fc Tag
|
|
|
Human
|
|
Human IL-6 R alpha / CD126 Protein, His-Tagged
|
|
|
Human
|
|
Biotinylated Human IL-6 R alpha / CD126 Protein, His-Tagged
|
|
|
Mouse
|
|
Mouse IL-6 R alpha / CD126 Protein, Fc Tag
|
|
|
VEGF
|
Human
|
|
Human VEGF165 protein, His tag
|
|
Human
|
|
Biotinylated human VEGF165 protein, no epitope tag, ultrasensitive, primary amine labeled
|
|
|
Human
|
|
Biotinylated human VEGF165 protein, His tag
|
|
|
Human
|
|
FITC labeled human VEGF165 protein, His tag
|
|
|
Human
|
|
Human VEGF121 protein, His tag
|
|
|
Human
|
|
Biotinylated human VEGF121 protein, His tag
|
|
|
Human
|
|
Human VEGF110 protein, His tag
|
|
|
Mouse
|
|
Mouse VEGF120 protein, untagged
|
|
|
TGF-beta
|
Human
|
|
Human TGF-Beta 1 / TGFB1 Protein
|
|
Human
|
|
Human TGF-Beta 1 / TGFB1 protein, untagged
|
|
|
Human
|
|
Biotinylated human TGF-Beta 1 / TGFB1 protein,
|
|
|
Mouse / Rat
|
|
Mouse/Rat TGF-Beta 1 / TGFB1 Protein, Untagged
|
|
|
Human
|
|
Human TGF-Beta 2 / TGFB2 protein, untagged
|
|
|
Human
|
|
Human Latent TGF-beta 3 / Latent TGFB3 Protein, His-Tagged
|
|
|
Human
|
|
Human LAP (TGF-beta 1) protein, His tag
|
|
|
Human
|
|
Human TGF-beta RII / TGFBR2 Protein, His-Tagged
|
|
|
Human
|
|
Biotinylated Human TGF-beta RII / TGFBR2 Protein, His, (HPLC verified)
|
|
|
Human
|
|
Human TGF-beta RII / TGFBR2 Protein, Llama IgG2b Fc Tag
|
|
|
CSF R
|
Human
|
HEK293
|
Biotinylated human M-CSF R / CSF1R / CD115 protein, His tag
|
|
Cynomolgus
|
HEK293
|
Cynomolgus monkey M-CSF R / CSF1R / CD115 protein, Fc tag
|
|
|
Cynomolgus
|
HEK293
|
Cynomolgus monkey M-CSF R / CSF1R / CD115 protein, His tag
|
|
|
Human
|
HEK293
|
Human M-CSF R / CSF1R / CD115 protein, mouse IgG2a Fc tag
|
|
|
Human
|
HEK293
|
Human M-CSF R / CSF1R / CD115 Protein, His-Tagged
|
|
|
Human
|
InsectCells
|
Human M-CSF R protein, His-tagged (active enzyme)
|
|
|
Human
|
HEK293
|
Human M-CSF R / CSF1R / CD115 Protein, Fc Tag, Low Endotoxin
|
|
|
Mouse
|
HEK293
|
Biotinylated mouse M-CSF R / CSF1R / CD115 protein, His tag
|
|
|
Mouse
|
HEK293
|
Mouse M-CSF R / CSF1R / CD115 Protein, His-Tagged
|
|
|
Mouse
|
HEK293
|
Mouse M-CSF R / CSF1R / CD115 Protein, Fc Tag, Low Endotoxin
|
|
|
Rabbit
|
HEK293
|
Rabbit M-CSF R/CSF1R/CD115 Protein, His-Tagged
|
|
|
Canine
|
HEK293
|
Canine M-CSF R / CSF1R / CD115 Protein, His-Tagged
|
|
|
Human
|
HEK293
|
Human G-CSF R/CD114 Protein, His-Tagged
|
|
|
Human
|
HEK293
|
Human G-CSF R/CD114 Protein, Fc Tag
|
|
|
Human
|
HEK293
|
Biotinylated human G-CSF R/CD114 protein, His tag
|
|
|
CSF
|
Human
|
HEK293
|
Human M-CSF Protein
|
|
Human
|
HEK293
|
Human M-CSF / CSF-1 Protein
|
|
|
Human
|
HEK293
|
Human M-CSF / CSF-1 Protein, His-Tagged
|
|
|
Human
|
HEK293
|
Biotinylated human M-CSF / CSF-1 protein, His tag
|
|
|
EGF
|
Human
|
|
Human EGF protein, His tag
|
|
Mouse
|
|
Mouse EGF protein, Fc tag
|
|
|
Human
|
|
Human EGF protein, mouse IgG2a Fc tag
|
|
|
EGF R
|
Human
|
|
Biotinylated human EGF R protein, His tag
|
|
Human
|
|
Biotinylated human EGF R protein, Fc tag
|
|
|
Human
|
|
APC labeled human EGF R protein, His tag
|
|
|
Human
|
|
Human EGF R protein, His tag, low endotoxin
|
|
|
Human
|
|
Human EGF R protein, Llama IgG2b Fc tag, low endotoxin
|
|
|
Human
|
|
FITC labeled human EGF R protein, Fc tag
|
|
|
Human
|
|
PE labeled human EGF R protein, His tag
|
|
|
Human
|
|
FITC labeled human EGF R protein, His tag
|
|
|
Human
|
|
Human EGF R protein, His tag
|
|
|
Human
|
|
Human EGF R protein, Fc tag
|
|
|
Mouse
|
|
Mouse EGF R protein, Fc tag
|
|
|
Mouse
|
|
Mouse EGF R protein, His tag
|
|
|
Mouse
|
|
Biotinylated mouse EGF R protein, His tag
|
|
|
Mouse
|
|
Biotinylated mouse EGF R protein, Fc tag
|
|
|
Human
|
|
Biotinylated human VEGF R2 / KDR protein, His tag
|
|
|
Human
|
|
Human VEGF R2 / KDR Protein, Fc Tag
|
|
|
Human
|
|
Human VEGF R2 / KDR Protein, Twin-Strep Tag
|
|
|
Human
|
|
PE labeled human VEGF R2 / KDR protein, His tag (site-specific binding)
|
|
|
Human
|
|
Human VEGF R2 / KDR Protein, His-Tagged
|
|
|
Human
|
|
FITC labeled human VEGF R2 / KDR protein, His tag
|
|
|
Complement C2
|
Human
|
|
Human complement C2 protein, His-tagged (active enzyme)
|
|
Cynomolgus
|
|
Biotinylated Cynomolgus Monkey Complement C2 Protein, His, (active enzyme, MALS verified)
|
|
|
Cynomolgus
|
|
Cynomolgus monkey complement C2 protein, His-tagged (active enzyme)
|
|
|
Rat
|
|
Biotinylated rat complement C2 protein, His-tag (active enzyme)
|
|
|
Rabbit
|
|
Biotinylated rabbit complement C2 protein, His-tag (active enzyme)
|
|
|
domestic pig
|
|
Biotinylated porcine complement C2 protein, His tag (active enzyme)
|
|
|
Dog
|
|
Biotinylated dog complement C2 protein, His-tag (active enzyme)
|
|
|
Human
|
|
Biotinylated human complement C3 protein, His tag
|
|
|
Human
|
|
Human complement C3 protein, His tag
|
|
|
Human
|
|
Human complement component 3 protein, Fc tag
|
|
|
Mouse
|
|
Mouse complement C3 protein, His tag
|
|
|
Cynomolgus
|
|
Cynomolgus monkey complement C3 protein, His tag
|
|
|
Complement C5
|
Human
|
|
Biotinylated human complement C5 protein, His tag
|
|
Human
|
|
Human Complement C5 (R885H) Protein, His-Tagged
|
|
|
Human
|
|
Human complement C5 protein, His tag
|
|
|
Human
|
|
Human complement C5 (w917s) protein, His-tagged
|
|
|
Human
|
|
Human complement C5 protein, Fc tag
|
|
|
Mouse
|
|
Mouse complement C5 protein, His tag
|
|
|
Cynomolgus
|
|
Cynomolgus complement C5 protein, His tag
|
|
|
Rat
|
|
Rat complement C5 protein, His tag
|
For more information about Seebio products, please call: 400-021-8158 / 021-50272975.
Application of cytokines in biopharmaceutical trials:
Cancer treatment: The use of cytokines in cancer treatment has been extensively studied. For example, immunostimulatory cytokines such as interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α), and interferon-α2 (IFN-α2) have been used in clinical trials and have shown some efficacy. However, the application of these cytokines is limited due to side effects and pharmacokinetic properties. Scientists are developing recombinant antibody-cytokine fusion proteins to improve the therapeutic index of cytokines.
Hepatotoxicity studies: In one study, the HepG2 cell line was used to study the toxic effects of drugs and cytokines on hepatocytes. In the experiment, cell death was assessed by detecting lactate dehydrogenase (LDH) release and caspase 3/7 activity after treatment with drugs and/or cytokines. In addition, quantitative sublethal hepatotoxicity imaging analysis was performed, using fluorescent probes to detect the content of cell nuclei, lipids, reactive oxygen species (ROS), mitochondrial membrane potential (MtMP), and glutathione (GSH). These data were used to generate a hepatotoxicity matrix through hierarchical cluster analysis to evaluate the effects of different drug and cytokine combinations on hepatotoxicity.
Applications of Cytokines in Biopharmaceutical Trials:
Cancer Treatment: The application of cytokines in cancer therapy has been extensively researched. Clinical trials have demonstrated some efficacy for immunostimulatory cytokines such as interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α), and interferon-α2 (IFN-α2). However, their use is constrained by side effects and pharmacokinetic limitations. To enhance the therapeutic index of cytokines, scientists are developing recombinant antibody-cytokine fusion proteins.
Hepatotoxicity Studies: A study employed the HepG2 cell line to investigate the toxic effects of drugs and cytokines on hepatocytes. The experiment assessed cell death by measuring lactate dehydrogenase (LDH) release and caspase 3/7 activity following treatment with drugs and/or cytokines. Additionally, quantitative sublethal hepatotoxicity imaging analysis was conducted using fluorescent probes to detect cell nucleus content, lipids, reactive oxygen species (ROS), mitochondrial membrane potential (MtMP), and glutathione (GSH) levels. These data were subjected to hierarchical cluster analysis to generate a hepatotoxicity matrix, which evaluated the impact of various drug and cytokine combinations on hepatocyte toxicity.
Vaccine adjuvants: Cytokines are used as vaccine adjuvants in the treatment of cutaneous melanoma. However, due to the potential for severe side effects, such as vascular leak syndrome, researchers have proposed the innovative idea of producing and applying "fusion factors". These fusion factors are hybrid molecules generated by cloning and fusing two different cytokine encoding cDNAs into a single open reading frame.
Adoptive cell therapy: In the treatment of B-cell malignancies, cytokines are administered to subjects who have a suboptimal response to administration of engineered cells (e.g., CAR-expressing cells) to improve the efficacy and/or anti-tumor activity of the administered cells. This suggests the potential use of cytokines in improving the efficacy of adoptive cell therapy.
Vaccine Adjuvants: Cytokines serve as adjuvants in vaccines aimed at treating cutaneous melanoma. Nevertheless, concerns over severe side effects, including vascular leak syndrome, have prompted researchers to propose an innovative concept: the production and application of "fusion factors." These fusion factors are hybrid molecules created by cloning and fusing two distinct cytokine-encoding cDNAs into a single open reading frame.
Adoptive Cell Therapy: In the management of B-cell malignancies, cytokines are administered to patients who exhibit a suboptimal response to the infusion of engineered cells (such as CAR-expressing cells) to augment the efficacy and/or antitumor activity of these cells. This underscores the potential role of cytokines in enhancing the effectiveness of adoptive cell therapy.
References:
1. Manfred Kopf , et al. Averting inflammation by targeting the cytokine environment. Nature Reviews Drug Discovery volume 9, pages703–718 (2010)
2. Thomas List, et al. Immunocytokines: a review of molecules in clinical development for cancer therapy. Clin Pharmacol. 2013 Aug 20;5(Suppl 1):29–45.
3. Benjamin D Cosgrove, et al. Synergistic Drug-Cytokine Induction of Hepatocellular Death. Toxicol Appl Pharmacol. 2009 Jun 15;237(3):317-30.
4. Agnieszka Sirko, et al. Recombinant cytokines from plants. Int J Mol Sci. 2011 Jun 3;12(6):3536–3552.
5. T. Albertson, et al. Treatment method using adoptive cell therapy. Patent number: CN113710256A
