Advanced Solution for C-Reactive Protein Detection through Fluorescence Immunochromatography
Time:2024-01-30 Hits:283
Project Background
"Guiding Principles for Technical Review of Registration of C-Reactive Protein Assay Kits."
C-Reactive Protein (CRP) is synthesized by liver cells and produced during the fetal period, transmitted non-maternally through the placenta. In response to infection or tissue damage, white blood cells produce interleukin-6 (IL-6), interleukin-1 (IL-1), and tumor necrosis factor Cytokines, which stimulate the liver to synthesize CRP. CRP is a disc-shaped polymer consisting of five non-covalently combined polypeptide chain subunits, with a molecular weight ranging from 115,000 to 140,000. It serves as a typical acute-phase protein.
Full C-reactive protein testing involves the simultaneous detection of conventional CRP and high-sensitivity CRP. Despite being the same substance, these differ in their quantitative lower limit of detection. Routine CRP measurement, encompassing qualitative, semi-quantitative, and quantitative analyses, aids in evaluating infection, tissue damage, and inflammatory diseases. Typically, a clinical level above 10 mg/L is considered for routine CRP determination.
Conventional CRP proves more sensitive and reliable than erythrocyte sedimentation rate (ESR) and white blood cell count in assessing acute inflammation. The linear range of high-sensitivity CRP is lower, allowing for expanded indications. CRP, being non-specific, requires comprehensive evaluation with clinical symptoms and is not a specific diagnostic basis.
High-sensitivity CRP is commonly used to aid in identifying cardiovascular disease risks. When used alongside traditional clinical diagnosis of acute coronary syndrome, it acts as an early warning indicator for the recurrence of coronary artery disease or acute coronary syndrome.
Performance
Fluorescence Immunochromatography (Lanthanide Eu Chelate Filling) Platform:
Components: Human CRP monoclonal antibody AP (Cat. No.: EKY0128AP) labeled fluorescent microspheres + human CRP monoclonal antibody AQ (Cat. No.: EKY0128AQ) for scratching.
Linear Range: 0.5mg/L-200mg/L.
Linear Range: 0.5mg/L-200mg/L.
Clinical Relevance
Fluorescence Immunochromatography (Lanthanide Eu Chelate Filling) Platform:
Clinical Correlation with Brand A CRP Reagent R2 > 0.95.
Correlation between the low-value part (≤40mg/L) and Brand A CRP Reagent R2 > 0.95.
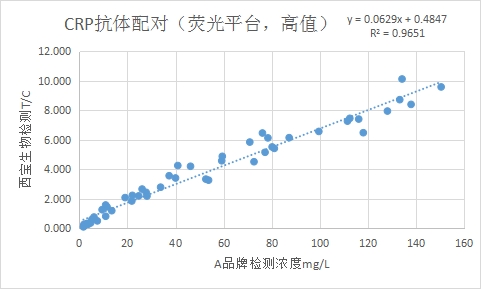
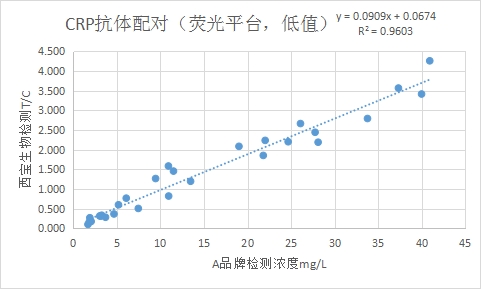
Test Program
Fluorescence Immunochromatography (Lanthanide Eu Chelate Filling) Platform:
Antigen to be tested: Human CRP antigen (Cat. No.: EKY0133B), Sample/Standard: Adding volume: 10ul.
Reaction Conditions: Mix pre-diluted sample + fluorescent solution, load sample, reaction time (10min-15min).
Fluorescence Instrument: Hemai FIC-S100; compatible with various fluorescence systems (excitation wavelength 365nm, emission wavelength 615nm) (e.g., Guangzhou Labsim, etc.).
Product Information
|
Product name
|
Item number
|
Recommended pairing
|
|
Human C-reactive protein (CRP) monoclonal antibody AP
|
Human C-reactive protein (CRP) monoclonal antibody AP (Cat. No.: EKY0128AP) labeled fluorescent microspheres + human C-reactive protein (CRP) monoclonal antibody AQ (Cat. No.: EKY0128AQ) (fluorescence immunochromatography combination)
|
|
|
Human C-reactive protein (CRP) monoclonal antibody AQ
|
|
|
|
Human C-reactive protein (CRP)
|
EKY0133B
|
|
|
Human C-reactive protein (CRP) monoclonal antibody AP-labeled fluorescent microsphere concentrate
|
DKY1305E
|
|
|
Time-resolved fluorescent microspheres, carboxyl (200nm)
|
AEG0786B
|
|
For more product details, please contact us: service@seebio.cn or Phone: +86 21 58183719 or Wechat: +86 158 0195 7578
