Photo Acid Generator (PAG)
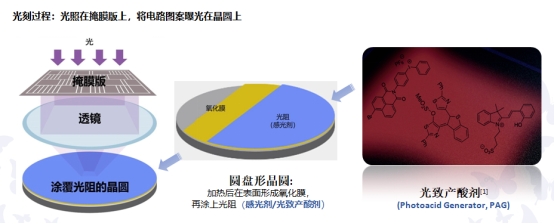
Seebio supplies Photo Acid Generator (PAG) 6-butyl-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl trifluoromethanesulfonate CAS# 1610827-31-0 with high quality in stock and bulk Package. The Photo Acid Generator (PAG) is a type of compound that decomposes under radiation, such as light, radiation, or plasma, to produce specific acids. These acids can cause acid-sensitive resins to degrade or crosslink, resulting in differential solubility between exposed and unexposed regions, ultimately forming an image after the development process. The Photo Acid Generator (PAG) is a fundamental component of photoresists and reacts to various forms of radiation, especially in the ultraviolet region.
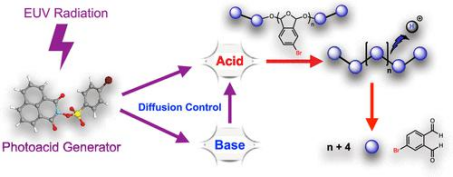
Chemical amplification of the photochemical reaction is illustrated [2].
Photoacid generators find extensive application and research in various imaging systems, including chemical amplification, anti-etch agents, and more. They are also widely used in cationic photocuring materials and thermally sensitive printing plates.
Main Types of Photoresists
| Product Type | Photolithography Wavelength | Semiconductor IC Process |
| g-line Photoresist | 436 nm | 0.5um |
| i-line Photoresist | 365 nm | 0.5-0.35um |
| KrF Photoresist | 248 nm | 250-130nm |
| ArF Photoresist (Dry) | 193 nm | 130-65nm |
| ArF Photoresist (Immersion) | 193 nm | 65-14nm, can reach 7nm with double and multiple patterning techniques |
| EUV Photoresist | 13.5 nm | Below 7nm |
| Electron Beam (e-beam) Photoresist System | Electron Beam | - |
Impact of Photoacids on Photoresist Performance:
Factors such as exposure time and the intensity of the light emitted by the light source need to be determined based on the characteristics of the photoacid. When formulating semiconductor photoresists, it is common to pair different photoacids with the resin design, and a single photoresist may contain more than one type of photoacid. Researchers add different photoacids based on the desired improvement direction for various parameters. The diffusion length of the photoacid directly determines the performance of the photoresist and critical parameters such as exposure energy, energy window, edge roughness, pattern morphology, and mask error enhancement factor.
An excellent photoacid should achieve the optimal balance between solubility, stability, sensitivity, and process window.
① High Sensitivity: The photoacid is highly sensitive to light, capable of generating a sufficient amount of acid at lower light intensities.
② Controllability: Different wavelengths of light sources can be chosen to activate the photoacid as needed, allowing precise control over the reaction.
N-Hydroxynaphthalimide Triflate Photo Acid Generator :
Chemical modification and tailoring of the naphthalimide structure are easily achievable and controllable. Naphthalimide triflate exhibits excellent light absorption properties and has high solubility in organic solvents, making it suitable for preparing polymeric compositions. It also possesses outstanding thermal stability and demonstrates good acid generation rates. These properties have made it a promising compound for photoacid generation since 2014. It holds significant research value and market potential.
Different chemical modifications allow for precise adjustments of the optical properties of 1,8-naphthalimide[3]:
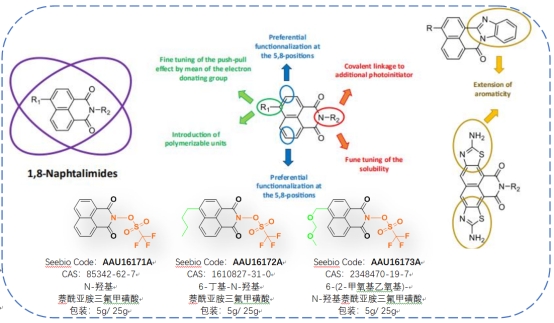
※ These compounds are mainly used in the manufacture of surface-modified polymer films and the preparation of positive-tone photoresists.
Through photolithography, patterned thin films are obtained for use in synthesizing photoresist compositions, producing composite materials, including substrates and coatings applied to the surface of substrates, among other applications [4].
Common Types of Photoacids
A wide variety of structurally diverse photoacid generators (PAGs) can generally be classified into two categories: ionic and non-ionic [5].
Ionic (Cationic) PAGs: These contain both cationic and anionic compounds. Widely used examples include diaryliodonium salts, triarylsulfonium salts, and those with anions such as BF4-, SbF6-, AsF6-, PF6-, or RSO3-. Of these, the triarylsulfonium salts are among the most commonly applied PAGs. The ironocene-based ionic compounds are also notable but have a relatively limited scope of application. Compared to triarylsulfonium salts, the acids produced by diaryliodonium salts have a higher quantum yield. Therefore, when a high acid generation rate is required, such as for manufacturing photocurable coatings, printing inks, and pressure-sensitive adhesives, these PAGs are preferred. On the other hand, the high thermal stability of triarylsulfonium salts is a primary factor in their use as PAGs in photoresists. Exposure to baking before and after exposure can compromise image quality if the cationic salt lacks sufficient thermal stability. Additionally, volatile iodine-substituted photoproducts generated by the photolysis of diaryliodonium salt-containing PAGs can deposit on the optical elements of equipment used for exposing photoresists. Photolysis of single triarylsulfonium-based PAGs does not lead to the formation of volatile photoproducts [6].
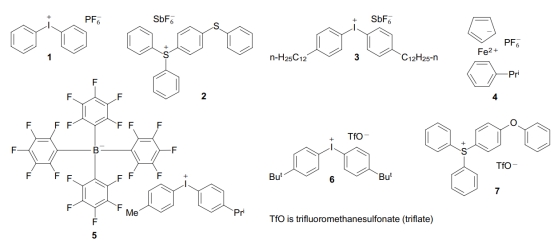
Commercial Ionic PAGs [5]
② Non-Ionic PAGs: Many non-ionic compounds are described in the literature that generates carboxylic acids, sulfonic acids, phosphoric acids, and hydrochloric acids when exposed to light. The most important compounds in this category are sulfonate esters, which produce strong acids upon exposure to light. These include arylsulfonate salts (Compound 8), acylimidesulfonate (Compound 9), and benzylsulfonate salts (Compound 10). Among the arylsulfonate salts, only para-nitro and ortho-nitro phenylsulfonate esters (Compound 11) generate acid upon exposure to light, and these isomers produce photoacids through different mechanisms. Non-ionic PAGs have relatively high solubility in organic solvents and polymer films, but they exhibit lower thermal stability compared to ionic salts (which can be improved through further chemical modifications).
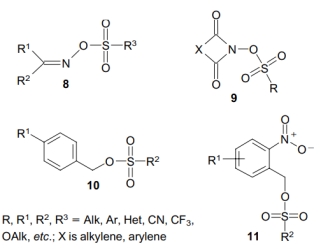
The main non-ionic PAG core structures can be found in the literature
[5].
Seebio offers a range of photo-initiators (PI), photosensitizers (PAC), and photoacid generators (PAG) products available in stock.
For product specifications and specific requirements, please contact: service@seebio.cn or Phone: +86 21 58183719
| Seebio Code | CAS No. | Chemical Name | Appearance | Purity | Absorption Peak (nm) |
| AAU16171A | 85342-62-7 | N-Hydroxynaphthalimide Triflate | / | ≥99% | / |
| AAU16172A | 1610827-31-0 | 6-butyl-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl trifluoromethanesulfonate | / | ≥99% | / |
| AAU16173A | 2348470-19-7 | 6-(2-methoxyethoxy)-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl trifluoromethanesulfonate | / | ≥99% | / |
| AAU16174A | 125051-32-3 | Bis(.eta.5-2,4-cyclopentadien-1-yl)- bis[2,6-difluoro-3-(1H-pyrrol-1-yl)phenyl]titanium | Yellow-Orange powder | ≥99% | 333, 396, 470 |
| AAU16175A | 162881-26-7 | Bis(2,4,6-trimethylbenzoyl) phenylphosphine oxide | Yellowish powder | ≥99% | 338, 370, 405 |
| AAU16176A | 75980-60-8 | 2,4,6-Trimethyl benzoyldiphenyl phosphine oxide | Yellowish powder | ≥99% | 295, 368, 380, 400 |
| AAU16177A | 84434-11-7 | Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate | Yellowish liquid | ≥98% | 369 |
| AAU16178A | 5495-84-1 | 2-Isopropylthioxanthone | Yellowish powder | ≥99% | 258, 382 |
| AAU16179A | 82799-44-8 | 2,4-Diethylthioxanthone | Yellowish powder | ≥99% | 261, 385 |
| AAU16180A | 71868-10-5 | 2-Methyl-1-[4-(methylthio)phenyl]-2- morpholinopropane-1-one | White powder | ≥99% | 240, 306 |
| AAU16181A | 119313-12-1 | 2-Benzyl-2-dimethylamino-1-(4- morpholinophenyl)butanone | Yellowish powder | ≥99% | 206, 323 |
| AAU16182A | 119344-86-4 | 2-Dimethylamino-2-(4-methyl-benzyl)- 1-(4-morpholin-4-yl-phenyl)-butan-1-one | Yellowish powder | ≥99% | 233, 320 |
| AAU16183A | 119-61-9 | Benzophenone | White flakes | ≥99.5% | 256, 330 |
| AAU16184A | 947-19-3 | 1-Hydroxy-Cyclohexyl Phenyl Ketone | White powder | ≥99% | 244, 280, 326 |
| AAU16185A | 7473-98-5 | 2-Hydroxy-2-methylpropiophenone | Colorless to pale yellow liquid | ≥99% | 244, 278, 322 |
| AAU16186A | 15206-55-0 | Methyl benzoylformate | Yellowish liquid | ≥99% | 255, 325 |
| AAU16187A | 24650-42-8 | 2,2-Dimethoxy-2-phenylacetophenone | Yellow-Brown powder | ≥98% | 300, 390, 450 |
| AAU16188A | 90-93-7 | 4,4'-Bis(diethylamino)benzophenone | Yellow Flakes | ≥98% | 244, 280, 330 |
| AAU16189A | 10287-53-3 | Ethyl-4-(dimethylamino)benzoate | White powder | ≥99% | |
| AAU16190A | / | Coumarin oxime ester | White powder | ≥98% | 297 |
| AAU16191A | 478556-66-0 | 1-[9-Ethyl-6-(2-methylbenzoyl)-9H-carbazol-3-yl]ethanone 1-(O-acetyloxime) | White powder | ≥99% | 252, 291, 328 |
| AAU16192A | 32760-80-8 | Cyclopentadienyliron(ii) hexa-fluorophosphate | Yellow-Brown powder | ≥98% | 300, 390, 450 |
| AAU16193A | 100011-37-8 | Cyclopentadienyliron(ii) hexa-fluoroantimonate | Yellow-Brown powder | ≥98% | 300, 390, 450 |
| AAU16194A | 344562-80-7 | 4-Isobutylphenyl-4'-methylphenyliodonium hexafluorophosphate | Yellowish liquid | 0.75 | 243 |
| AAU16195A | 71786-70-4 | Bis(4-dodecylphenyl)iodonium hexaflurorantimonate | Yellowish liquid | 0.5 | 278 |
| AAU16196A | 61358-25-6 | Bis(4-tert-butylphenyl)iodonium hexafluorophosphate | White powder | ≥99% | 265 |
| AAU16197A | 42573-57-9 | 2-[2-(4-Methoxyphenyl-2-yl)vinyl]-4,6-bis(trichloromethyl)1,3,5-triazine | Yellow powder | ≥98% | 249, 373 |
| AAU16198A | / | p-methoxyphenylacetonitrile, a-[5-[[(p-toluene sulfonyl)oxy]imino]-2(5H)-thienylidene] | Yellow-Brown powder | ≥97% | 270, 430 |
| AAU16199A | 506426-96-6 | 2,4-Oxazolidinedione, 5-[[4-(diphenylamino)phenyl]methylene]-3-(2-phenylethyl)- | Yellow powder | ≥99% | 395 |
References:
[1]. Zivic Z. et al, Angew. Chem. Int. Ed. 2019, 58, 10410 – 10422.
[2]. Deng J.Y. et al, Chemistry of Materials 2022
[3]. Noirbent G. et al, European Polymer Journal 2020, 132,109702.
[4]. K.W. et al, WO2019/ 045377 KO 2019 .03 .07
[5]. N.A.Kuznetsova, G.V.Malkov, B.G.GribovRuss. Chem. Rev., 2020, 89, 2,173-190.
[6]. Reichmanis E. et al, J.Bowden. Mat. Res. Soc. Symp. Proc., 2000, 23, 584.
[7]. Martin C.J. et al, J. Photochem. Photobiol., C, 2018, 34, 41.
