How to choose Amidation Reagents?
Time:2023-09-28 Hits:463
The acyl amide condensation reaction is a commonly used and mild method for preparing amide bonds, requiring amide bond formation reagents to activate carboxylic acids during the process. Such reactions are also widely applied in the construction of ester bonds, macrocyclic amides, and lactones.
With the rapid development of peptide-related applications and the increasing scale of industrial production, the use of efficient amide bond formation reagents for peptide synthesis has become a research focus in the field of peptide development. Currently, peptide synthesis primarily utilizes activated carboxyl groups to connect peptides in reactions. The choice of condensation reagent is crucial in peptide synthesis, as the correct selection can significantly enhance experimental efficiency. Seebio can provide peptide condensation reagents. For inquiries, please contact us at service@seebio.cn.
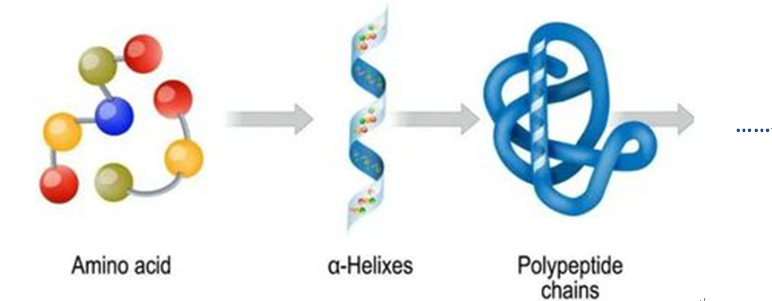
Peptide Synthesis
1. General reaction mechanism:
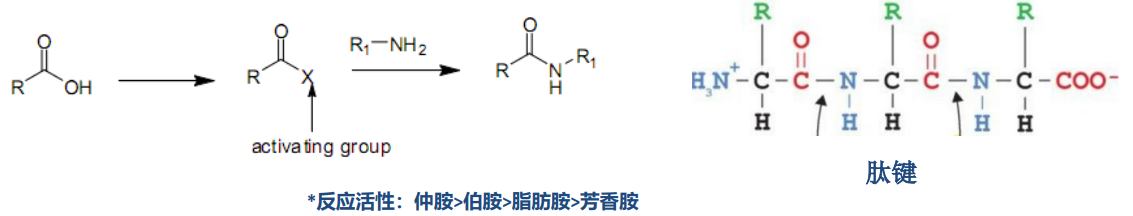
Reactivity sequence Secondary amines > Primary amines > Aliphatic amines > Aromatic amines
2. Cationic Salt-Based Coupling Agent Method

① Carbodiimide Condensing Agent Method
DCC, DIC, EDC, BDC are widely used in carbodiimide (-N=C=N-) condensation agent which are suitable for large-scale preparation of polypeptides. The resin-mounted EDC (P-EDC) solidifies the condensation agent on the polymer, making the post-treatment of the reaction simpler.

② Cationic Salt-Based Coupling Agent Method
Cationic salts of coupling agents exhibit high activity. Common urea cationic salts, such as HATU (excellent coupling efficiency but relatively expensive), HBTU, and phosphonium cationic salts like PyBOP (high activity, considered when other methods do not work), and BOP.
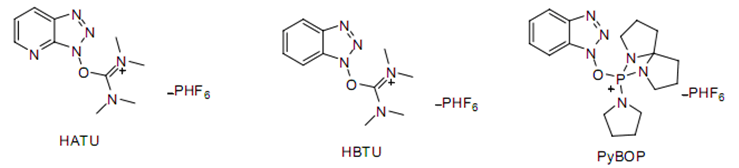
Urea salt
Advantages such as fast reaction rates, minimal racemization of products, and high yields, coupling agents based on HOBt and HOAt can effectively facilitate the formation of sterically hindered amide bonds. Maturely applied in the market are TBTU, HATU, PyCIU, TFFH, BTFFH, CIP, and CTDP.
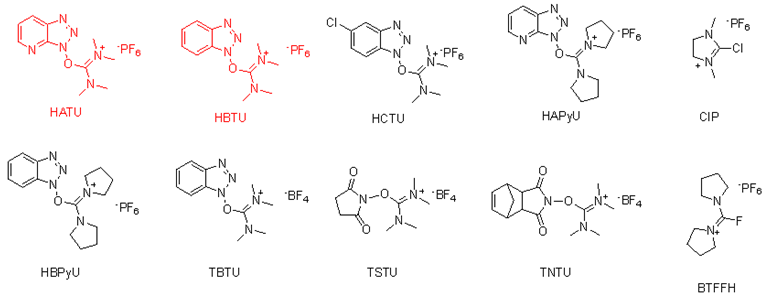
Common ureapillium reagents
Phosphorium salt
Phosphorium salt can promote the formation of sterically hindered peptide amide bonds and is commonly used in the synthesis of peptides containing N-methyl amino acids or C, C-dialkylated amino acids, as well as biologically active peptides.
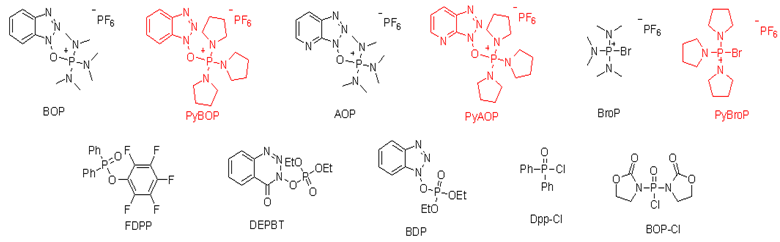
Common phosphognium reagents
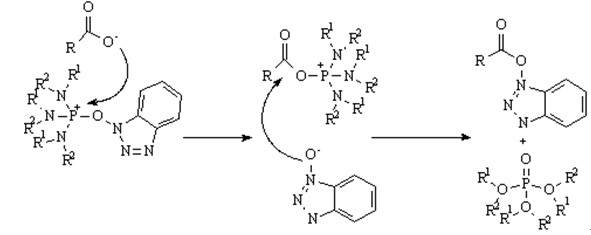
HOBt-based Phosphonium Salt Mediated Coupling
③ CDI Active Ester Coupling Method
CDI is used as a formyl transfer reagent and for the activation of carboxylic acids. The coupling is carried out in two steps. First, CDI reacts with the carboxylic acid to generate a highly active acyl imidazole. Subsequently, the amine readily reacts with this highly active ester to form the corresponding acylation product. It is crucial to strictly control the amounts of CDI and amine, maintaining an equivalent ratio of 1 (to avoid excess CDI and amine, which can produce urea byproducts).
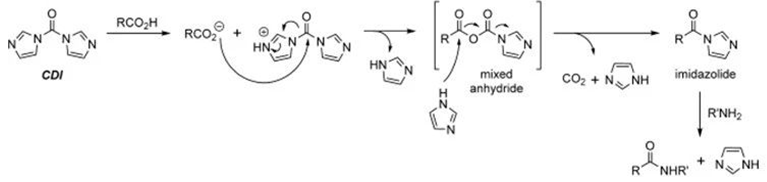
CDI condensation process
3.Selection of Bases:
TEA and DIEA are commonly used bases in amide bond formation. For chiral reactions, TEA, as reported in the literature, may result in racemization, while the likelihood of racemization is much lower with DIEA.
For phenol-based amide bond formation, the use of inorganic bases (such as K2CO3) can help avoid ester formation.
4 General Guidelines for Peptide Coupling Reactions:
Start by selecting TBTU and HOBT, EDCI in that order. If the reaction yields unsatisfactory results, consider trying HATU, PyBOP (or PyAOP), and PyBroP.
If none of the above coupling agents provide the desired results, CDI can be used as an alternative. Dissolve the acid in CH2Cl2, add CDI in portions, and after the reaction is complete, transfer it to a dropping funnel. Drip this mixture into the CH2Cl2 solution of the amine, which typically yields good results and is straightforward to execute.
If none of the previous methods work, you can convert the acid into an acid chloride and react it with the amine under triethylamine conditions to form the amide.
Finally, consider using carboxylic acid salts for the coupling reaction with the amine.
*Some of the article content and pictures come from the Internet. *
Seebio offers commercial acidamine condensation reagents, for specifications and requirements, please contact:service@seebio.cn.
|
Seebio Code
|
Product name
|
CAS
|
categorization
|
|
AAU16001A
|
N,N'-Dicyclohexylcarbodiimide(DCC)
|
538-75-0
|
Carbodiimide type
|
|
AAU16002A
|
N,N'-Diisopropylcarbodiimide(DIC)
|
693-13-0
|
|
|
AAU16005A
|
O-(2-Mesitylenesulfonyl)acethydroxamic acid ethyl ester(HOOBt)
|
28230-32-2
|
|
|
AAU16006A
|
6-Chloro-1-hydroxibenzotriazol(Cl-HOBt)
|
26198-19-6
|
|
|
AAU16007A
|
N-Hydroxy-7-azobenzotriazole(HOAt)
|
39968-33-7
|
|
|
AAU16008A
|
N-Hydroxysuccinimide(HOSu)
|
6066-82-6
|
|
|
AAU16009A
|
1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate(BOP)
|
56602-33-6
|
|
|
AAU16010A
|
Benzotriazole-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate(PyBOP)
|
128625-52-5
|
|
|
AAU16011A
|
(3-Hydroxy-3H-1,2,3-triazolo[4,5-b]pyridinato-O)tri-1-pyrrolidinylphosphonium hexafluorophosphate(PyAOP)
|
156311-83-0
|
Phosphorus positive ionic type
|
|
AAU16012A
|
Bromo-tris-pyrrolidino-phosphonium hexafluorophosphate(PyBrOP)
|
132705-51-2
|
|
|
AAU16013A
|
2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate(TBTU)
|
125700-67-6
|
|
|
AAU16014A
|
2-(7-Azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate(HATU)
|
148893-10-1
|
|
|
AAU16015A
|
2-(7-Azabenzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate(TATU)
|
873798-09-5
|
Urea positive ionic type
|
|
AAU16016A
|
O-(6-Chlorobenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate(TCTU)
|
330641-16-2
|
|
|
AAU16017A
|
O-Benzotriazole-N,N,N',N'-tetraMethyl-uroniuM-hexafluorophosphate(HBTU)
|
94790-37-1
|
|
|
AAU16018A
|
5-Chloro-1-[bis(dimethylamino)methylene]-1H-benzotriazolium 3-oxide hexafluorophosphate(HCTU)
|
330645-87-9
|
|
|
AAU16019A
|
N,N,N',N'-Tetramethyl-O-(3,4-dihydro-4-oxo-1,2,3-benzotriazin-3-yl)uronium tetrafluoroborate(TDBTU)
|
125700-69-8
|
|
|
AAU16020A
|
Fluoro-N,N,N',N'-tetramethylformamidinium hexafluorophosphate(TFFH)
|
164298-23-1
|
|
|
AAU16021A
|
2-SucciniMido-1,1,3,3-tetra-methyluronium tetrafluoroborate(TSTU)
|
105832-38-0
|
|
|
AAU16022A
|
1,1'-Carbonyldiimidazole(CDI)
|
530-62-1
|
|
|
AAU16023A
|
Bis(2-oxo-3-oxazolidinyl)phosphinic chloride(BOP-Cl)
|
68641-49-6
|
|
|
AAU16024A
|
Diphenylphosphoryl azide(DPPA)
|
26386-88-9
|
Other
|
|
AAU16025A
|
4-Dimethylaminopyridine(DMAP)
|
1122-58-3
|
|
|
AAU16026A
|
4,5-Dicyanoimidazole
|
1122-28-7
|
|
|
AAU16027A
|
N-Ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline(EEDQ)
|
16357-59-8
|
For commonly used amide reagents, please contact:service@seebio.cn.
|
Seebio Code
|
Product name
|
CAS
|
Purity
|
Package
|
|
AAU16028A
|
2,4,6-Triisopropylbenzenesulfonyl chloride(TPSCL)
|
6553-96-4
|
99%
|
5g; 25g; 100g
|
|
AAU16029A
|
4-Nitrophenyl trifluoroacetate(TFAONP)
|
658-78-6
|
99%
|
5g; 25g; 100g
|
|
AAU16030A
|
1,8-Diazabicyclo[5,4,0]undec-7-ene(DBU)
|
6674-22-2
|
99%
|
5g; 25g; 100g
|
|
AAU16032A
|
Methyl 4-chlorocarbonylbenzoate(MMT-CL)
|
7377-26-6
|
99%
|
5g; 25g; 100g
|
|
AAU16033A
|
N,N'-Disuccinimidyl carbonate(DSC)
|
74124-79-1
|
99%
|
5g; 25g; 100g
|
|
AAU16034A
|
1-(Mesitylene-2-sulfonyl)-3-nitro-1,2,4-triazole(MSNT)
|
74257-00-4
|
99%
|
5g; 25g; 100g
|
|
AAU16035A
|
Pentafluorophenol(PFP-OH)
|
771-61-9
|
99%
|
5g; 25g; 100g
|
|
AAU16036A
|
1-(p-Toluenesulfonyl)-3-nitro-1,2,4-triazole(TSNT)
|
77451-51-5
|
99%
|
5g; 25g; 100g
|
|
AAU16037A
|
1,1'-Carbonyldipyrrolidine(CDP)
|
81759-25-3
|
99%
|
5g; 25g; 100g
|
|
AAU16038A
|
1-(4-Methylphenylsulfonyl)-1,2,4-triazole(MPST)
|
13578-51-3
|
99%
|
5g; 25g; 100g
|
|
AAU16039A
|
Chloro-dipiperidinocarbenium hexafluorophosphate(PipClU)
|
161308-40-3
|
99%
|
5g; 25g; 100g
|
|
AAU16040A
|
Benzotriazole-1-carboxaMidinium tosylate(BCAT)
|
163853-10-9
|
99%
|
5g; 25g; 100g
|
|
AAU16041A
|
O-(3,4-Dihydro-4-oxo-1,2,3-benzotriazin-3-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate(HDBTU )
|
164861-52-3
|
99%
|
5g; 25g; 100g
|
|
AAU16042A
|
N,N,N',N'-Tetramethyl-S-(1-oxido-2-pyridyl)thiuronium hexafluorophosphate(HOTT)
|
212333-72-7
|
99%
|
5g; 25g; 100g
|
|
AAU16043A
|
[Dimethylamino-(2-oxopyridin-1-yl)oxymethylidene]-dimethylazanium,hexafluorophosphate(HPTU)
|
364047-51-8
|
99%
|
5g; 25g; 100g
|
|
AAU16044A
|
Isobutyl 1,2-dihydro-2-isobutoxy-1-quinoline-carboxylate(IIDQ)
|
38428-14-7
|
99%
|
5g; 25g; 100g
|
|
AAU16045A
|
Mesitylenesulfonyl-1H-1,2,4-triazole(MTST)
|
54230-59-0
|
99%
|
5g; 25g; 100g
|
|
AAU16046A
|
Triisopropylsilane(TIPS)
|
6485-79-6
|
99%
|
5g; 25g; 100g
|
|
AAU16047A
|
9-FluorenylMethyl pentafluorophenyl carbonate(Fmoc-OPFP)
|
88744-04-1
|
99%
|
5g; 25g; 100g
|
|
AAU16048A
|
Ethyl 1-hydroxy-1H-1,2,3-triazole-4-carboxylate(HOCT)
|
137156-41-3
|
99%
|
5g; 25g; 100g
|
|
AAU16049A
|
(Benzotriazol-1-yloxy)dipiperidinocarbenium hexafluorophosphate(HBPipU)
|
190849-64-0
|
99%
|
5g; 25g; 100g
|
|
AAU16050A
|
2-(1-Oxy-pyridin-2-yl)-1,1,3,3-tetramethylisothiouronium tetrafluoroborate(TOTT)
|
255825-38-8
|
99%
|
5g; 25g; 100g
|
|
AAU16051A
|
O-Succinimidyl-1,3-dimethylpropyleneuronium hexafluorophosphate(HPD-Osu)
|
443305-33-7
|
99%
|
5g; 25g; 100g
|
|
AAU16052A
|
N-MesitylenesulfonyliMidazole
|
50257-39-1
|
99%
|
5g; 25g; 100g
|
|
AAU16053A
|
N,N,N',N'-Tetramethylchloroformamidinium hexafluorophosphate(TCFH)
|
94790-35-9/207915-99-9
|
99%
|
5g; 25g; 100g
|
|
AAU16054A
|
1,3,5,2,4,6-Trioxatriphosphorinane, 2,4,6-tributyl-, 2,4,6-trioxide(50% Ethyl acetate solution)
|
163755-62-2
|
99%
|
5g; 25g; 100g
|
|
AAU16055A
|
1-(3-DiMethylaMinopropyl)-3-ethylcarbodiiMide Methiodide
|
22572-40-3
|
99%
|
5g; 25g; 100g
|
|
AAU16056A
|
O-SucciniMidyl-1,3-diMethylpropyleneuroniuM tetrafluoroborate(TPD-Osu)
|
443305-34-8
|
99%
|
5g; 25g; 100g
|
|
AAU16057A
|
S-(1-Oxo-2-pyridyl)-thio-1,3-dimethylpropyleneuronium hexafluorophosphate(HPTDP)
|
366821-62-7
|
99%
|
5g; 25g; 100g
|
|
AAU16058A
|
1-(4-Nitrobenzenesulfonyl)-1H-1,2,4-triazole(NBST)
|
57777-84-1
|
99%
|
5g; 25g; 100g
|
Related Products
