SNAC:Medication Oral Absorption Enhancer
Time:2023-11-07 Hits:292
8-(2-Hydroxybenzoylamino)octanoic acid sodium (SNAC, Salcaprozate sodium) can serve as an oral absorption enhancer and has gained considerable attention in the delivery of orally administered heparin and insulin. Salcaprozate sodium has the potential to increase lipophilicity induced by non-covalent large-molecule complexes, thereby enhancing passive transcellular permeation across intestinal epithelial cells [1][2].
The Israeli company Chiasma has established a TPE (Transient Permeability Enhancer) absorption enhancement platform based on the characteristics of sodium octanoate. They successfully prepared an oral form of octreotide using this technology. The formulation consists of medium-chain fatty acids, sodium octanoate, and other excipients. Octreotide binds to the oil-suspension formulation, which is then encapsulated in a pH-dependent polymer enteric coating. In June 2020, the U.S. FDA approved the marketing of octreotide oral capsules (Mycapssa), a somatostatin analog, for long-term maintenance therapy in patients with acromegaly. Sodium octanoate can alter the gastrointestinal membrane's flow properties and increase protein release within cells, thereby enhancing cellular permeability [4]. Seebio can provide reagent-grade and pharmaceutical-grade 8-(2-Hydroxybenzoylamino)octanoic acid sodium (SNAC, Salcaprozate sodium).
For more product details, please contact us: service@seebio.cn or Phone: +86 21 58183719 or Wechat: +86 158 0195 7578
Basic Information:
Medication Oral Absorption Enhancer - SNAC
Product Name: 8-(2-Hydroxybenzoylamino)octanoic acid sodium (SNAC)
English Name: Salcaprozate sodium
CAS No.: 203787-91-1
Molecular Weight: 301.32
Molecular Formula: C15H20NNaO4
Purity: 98.0% to 102.0% on the anhydrous basis
Preparation of stock solution: Solvent is DMSO at 50 mg/ml (165.94 mM; requires ultrasonication).
Product Name: 8-(2-Hydroxybenzoylamino)octanoic acid sodium (SNAC)
English Name: Salcaprozate sodium
CAS No.: 203787-91-1
Molecular Weight: 301.32
Molecular Formula: C15H20NNaO4
Purity: 98.0% to 102.0% on the anhydrous basis
Preparation of stock solution: Solvent is DMSO at 50 mg/ml (165.94 mM; requires ultrasonication).
Preparation of Stock Solution
Solvent: DMSO : 50 mg/ml (165.94 mM; Need ultrasonic).
|
Mass
Solvent Volume Concentration |
1 mg
|
5 mg
|
10 mg
|
|
1 mM
|
3.3188 ml
|
16.5942 ml
|
33.1884 ml
|
|
5 mM
|
0.6638 ml
|
3.3188 ml
|
6.6377 ml
|
|
10 mM
|
0.3319 ml
|
1.6594 ml
|
3.3188 ml
|
*The solubility of the product may vary in different solvents. Please choose the appropriate solvent for dissolution. Avoid repeated freeze-thaw cycles to maintain product effectiveness.
Conversion of Equivalent Doses in Different Experimental Animals Based on Body Surface Area [3]
Animal A (mg/kg) = Animal B (mg/kg) × Animal B's Km Factor / Animal A's Km Factor
For example, based on the body surface area conversion method, to convert a dose of 20 mg/kg for mice to an equivalent dose for rats, you need to multiply 20 mg/kg by the Km factor for mice and then divide by the Km factor for rats, resulting in an equivalent dose of 10 mg/kg for rats.
|
Animal
Program |
Mouse
|
Rat
|
Rabbit
|
Guinea Pig
|
Hamster
|
Dog
|
|
Weight (kg)
|
0.02
|
0.15
|
1.8
|
0.4
|
0.08
|
10
|
|
Body Surface Area (m2)
|
0.007
|
0.025
|
0.15
|
0.05
|
0.02
|
0.5
|
|
Km Factor
|
3
|
6
|
12
|
8
|
5
|
20
|
Product List
|
Catalog Number
|
Product
|
Grade
|
Packaging
|
|
AJL1314A
|
Sodium (2-hydroxybenzamide) octanoate (SNAC)
CAS: 203787-91-1
|
Reagent Grade
|
250mg、500mg、1g、5g
|
|
AJL1314B
|
Pharmaceutical Grade
|
500g、1Kg、25Kg
|
Research Areas:
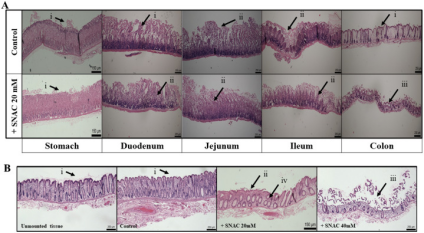
Minor intestinal mucosal changes with SNAC in KH:In a 120-minute study, minor changes in the structure of intestinal mucosae were observed when exposed to SNAC in KH buffer. Rat stomach mucosae remained structurally intact with slight disruptions when exposed to 20 mM SNAC. However, in rat duodenal and jejunal mucosae, minor cell sloughing at the tips of villi was seen in both control and SNAC-exposed tissues. More substantial sloughing occurred in rat ileal and colonic mucosae when exposed to SNAC, leading to the absence of villi tips.
Human colonic mucosae in KH buffer appeared healthy and structurally intact, similar to control tissue. When exposed to 20 mM SNAC, minor damage to the villi tips was observed, while 40 mM SNAC caused tip erosion in human tissue[5].
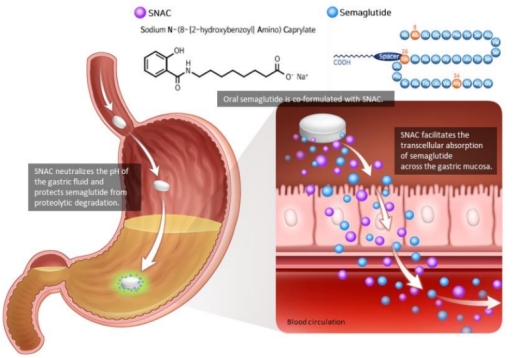
SNAC as a Medication Oral Absorption Enhancer:To enable oral delivery, semaglutide is co-formulated with the absorption enhancer sodium N-(8-[2-hydroxybenzoyl])amino) caprylate (SNAC), which protects it from degradation and promotes absorption across the stomach. Semaglutide requires injection due to rapid enzymatic degradation and low GI tract pH. However, when combined with SNAC, semaglutide can be absorbed orally.
SNAC increases semaglutide's lipophilicity and transcellular absorption through the stomach in a concentration-dependent manner by temporarily binding to it. Additionally, SNAC acts as a local pH buffer, increasing solubility and protecting against degradation. Once absorbed, SNAC readily dissociates from semaglutide. Thus, SNAC enables oral delivery by protecting and promoting semaglutide's absorption[6].
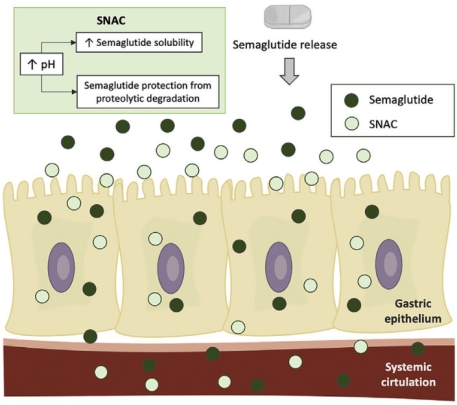
The Eligen technology enables oral delivery of semaglutide using the absorption enhancer SNAC to prevent degradation and improve bioavailability. Semaglutide normally requires injection due to rapid enzymatic degradation and low GI tract pH. However, when combined with SNAC in the formulation Rybelsus, semaglutide can be absorbed orally. SNAC increases local pH, allowing semaglutide to utilize passive transcellular transport across the gastric epithelium in a time- and concentration-dependent manner. This results in approximately 0.4-1% bioavailability for oral Rybelsus. Thus, SNAC protects semaglutide from degradation and promotes absorption across the stomach to overcome barriers to oral delivery[7].
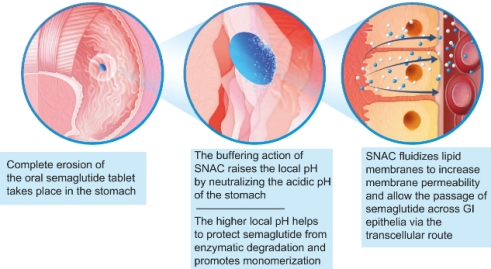
Mode of action of SNAC in oral semaglutide: SNAC enables oral delivery of semaglutide by temporarily increasing gastric pH and fluidizing the plasma membrane to promote transcellular absorption. When co-formulated, SNAC provides buffering action that increases local pH as the tablet erodes, protecting semaglutide from enzymatic degradation. SNAC also promotes monomerization by changing solution polarity. Additionally, SNAC incorporates into the lipid membrane of gastric cells, fluidizing the plasma membrane and allowing semaglutide to transit transcellularly. These effects are reversible, with the membrane returning to its solid state after transporting semaglutide. Thus, SNAC promotes absorption by transiently modifying the gastric environment without permanent disruption[8].
References:
[1]. Riley MGI, et, al. Subchronic oral toxicity of salcaprozate sodium (SNAC) in Sprague-Dawley and Wistar rats. Int J Toxicol. Jul-Aug 2009; 28(4):278-93.
[2]. Twarog C, et, al. Intestinal Permeation Enhancers for Oral Delivery of Macromolecules: A Comparison between Salcaprozate Sodium (SNAC) and Sodium Caprate (C 10). Pharmaceutics. 2019 Feb 13; 11(2):78.
[3]. Anroop B. Nair, Shery Jacob.A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. March 2016-May 2016; 7(2): 27-31.
[4]. Song Bo. Research Progress on the Application and Development of Absorption Enhancers for Oral Drugs [J]. China Pharmaceutical Industry, 2022, 31(22): 127-132.
[5]. Sarinj Fattah. Salcaprozate sodium (SNAC) enhances permeability of octreotide across isolated rat and human intestinal epithelial mucosae in Ussing chambers. https://doi.org/10.1016/j.ejps.2020.105509
[6]. Hwi Seung Kim. Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes.DOI:10.3390/ijms22189936
[7]. Carlos Bendicho Lavilla. Fighting type 2 diabetes: Formulation strategies for peptide-based therapeutics. DOI:10.1016/j.apsb.2021.08.003
[8]. Vanita R. Aroda. A new era for oral peptides: SNAC and the development of oral semaglutide for the treatment of type 2 diabetes. https://doi.org/10.1007/s11154-022-09735-8
[2]. Twarog C, et, al. Intestinal Permeation Enhancers for Oral Delivery of Macromolecules: A Comparison between Salcaprozate Sodium (SNAC) and Sodium Caprate (C 10). Pharmaceutics. 2019 Feb 13; 11(2):78.
[3]. Anroop B. Nair, Shery Jacob.A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. March 2016-May 2016; 7(2): 27-31.
[4]. Song Bo. Research Progress on the Application and Development of Absorption Enhancers for Oral Drugs [J]. China Pharmaceutical Industry, 2022, 31(22): 127-132.
[5]. Sarinj Fattah. Salcaprozate sodium (SNAC) enhances permeability of octreotide across isolated rat and human intestinal epithelial mucosae in Ussing chambers. https://doi.org/10.1016/j.ejps.2020.105509
[6]. Hwi Seung Kim. Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes.DOI:10.3390/ijms22189936
[7]. Carlos Bendicho Lavilla. Fighting type 2 diabetes: Formulation strategies for peptide-based therapeutics. DOI:10.1016/j.apsb.2021.08.003
[8]. Vanita R. Aroda. A new era for oral peptides: SNAC and the development of oral semaglutide for the treatment of type 2 diabetes. https://doi.org/10.1007/s11154-022-09735-8
Related Products
